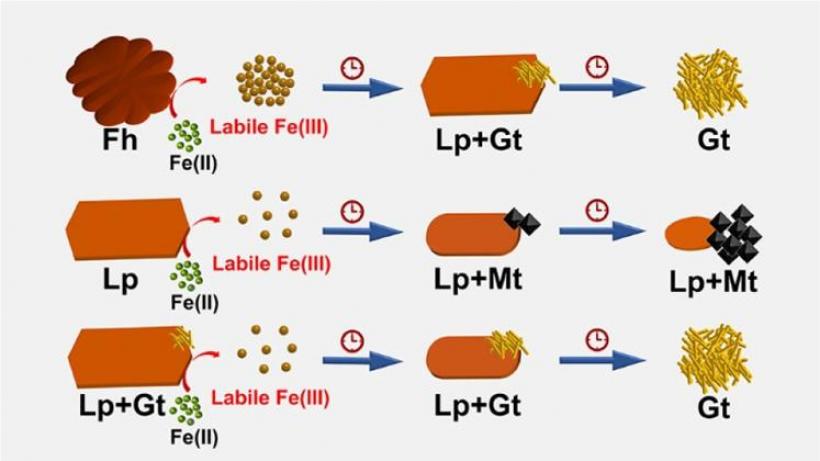
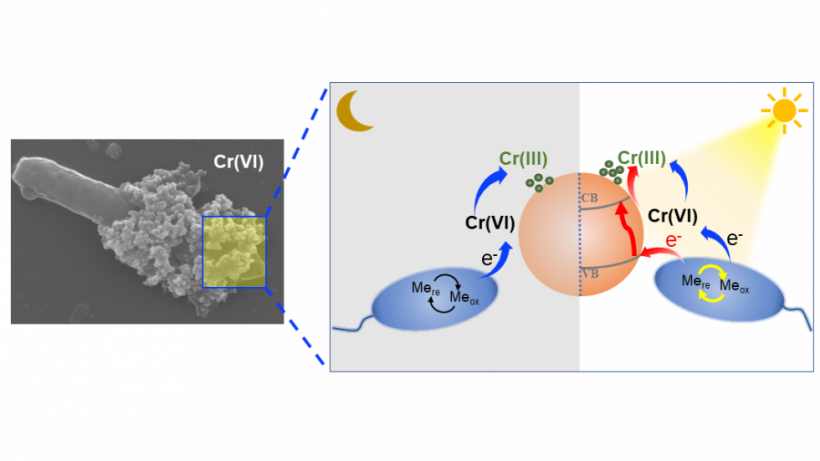
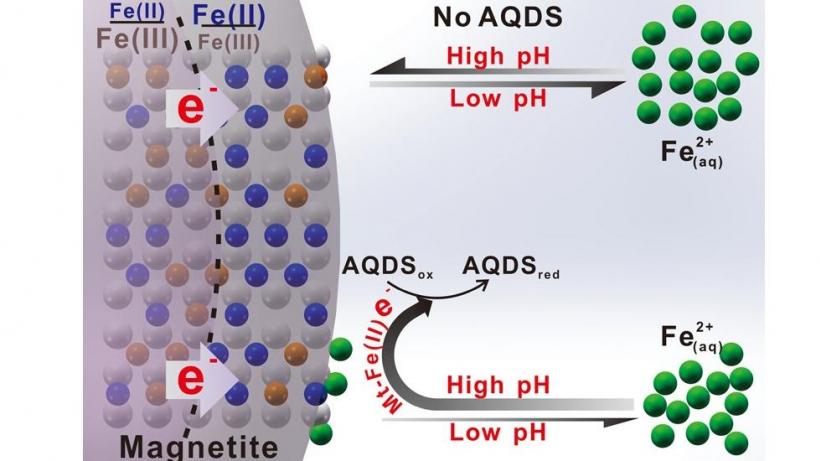
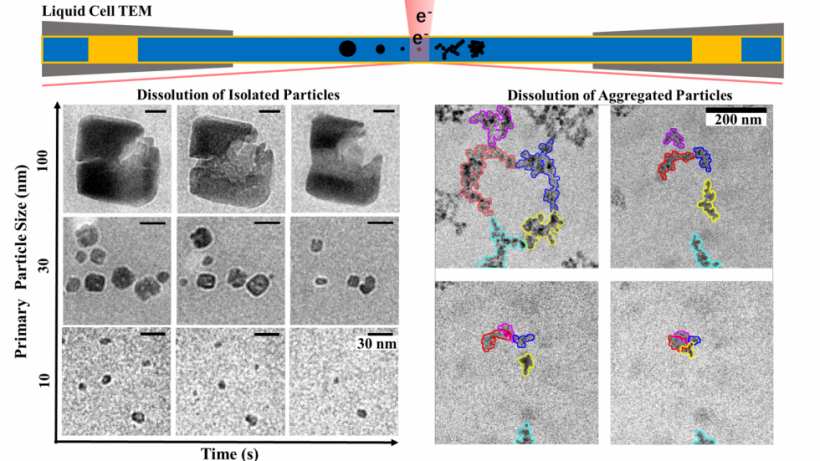
Zhong C, Lu A, Dong H, Huang S, Shi L, Shen Y, Cheng Y, Dong Y, Li X, Xu J, et al. Photoelectron-promoted metabolism of sulphate-reducing microorganisms in substrate-depleted environments. Environmental Microbiology [Internet]. 2024. 访问链接Abstract
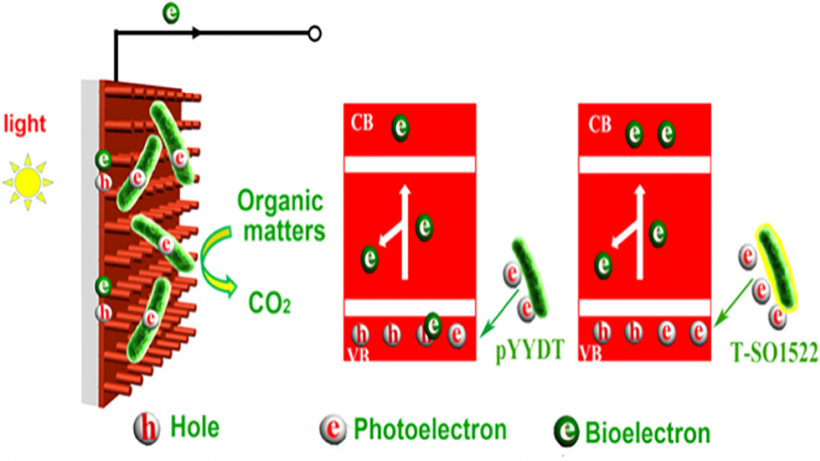
Sulphate-reducing microorganisms, or SRMs, are crucial to organic decomposition, the sulphur cycle, and the formation of pyrite. Despite their low energy-yielding metabolism and intense competition with other microorganisms, their ability to thrive in natural habitats often lacking sufficient substrates remains an enigma. This study delves into how Desulfovibrio desulfuricans G20, a representative SRM, utilizes photoelectrons from extracellular sphalerite (ZnS), a semiconducting mineral that often coexists with SRMs, for its metabolism and energy production. Batch experiments with sphalerite reveal that the initial rate and extent of sulphate reduction by G20 increased by 3.6 and 3.2 times respectively under light conditions compared to darkness, when lactate was not added. Analyses of microbial photoelectrochemical, transcriptomic, and metabolomic data suggest that in the absence of lactate, G20 extracts photoelectrons from extracellular sphalerite through cytochromes, nanowires, and electron shuttles. Genes encoding movement and biofilm formation are upregulated, suggesting that G20 might sense redox potential gradients and migrate towards sphalerite to acquire photoelectrons. This process enhances the intracellular electron transfer activity, sulphur metabolism, and ATP production of G20, which becomes dominant under conditions of carbon starvation and extends cell viability in such environments. This mechanism could be a vital strategy for SRMs to survive in energy-limited environments and contribute to sulphur cycling.