欢迎报考2026级环境科学博士生
五月 28, 2025
欢迎全国2022级优秀本科生推免本研究组2026级博士生!
College of Environmental Sciences and Engineering, Peking University, Beijing 100871, China
zmchen@pku.edu.cn
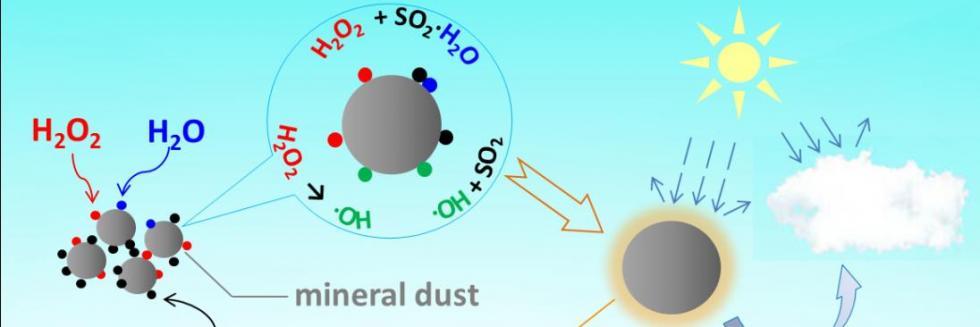
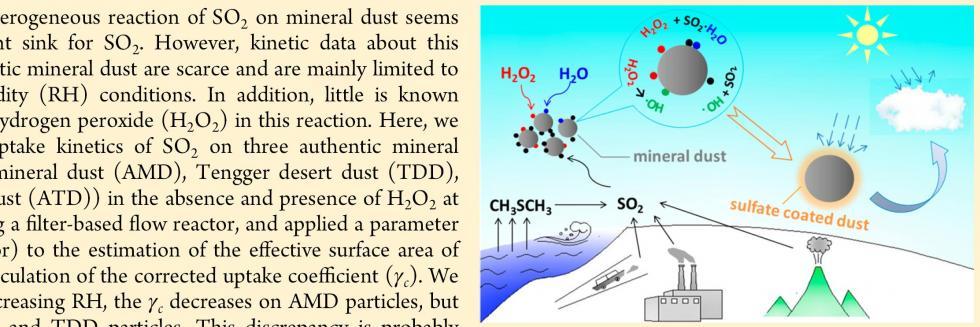
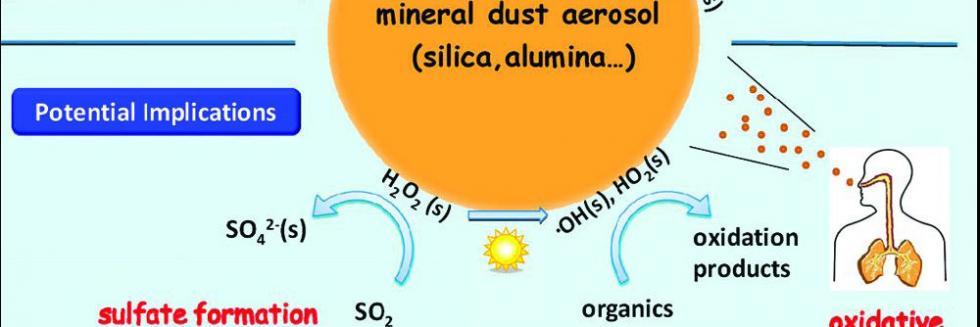
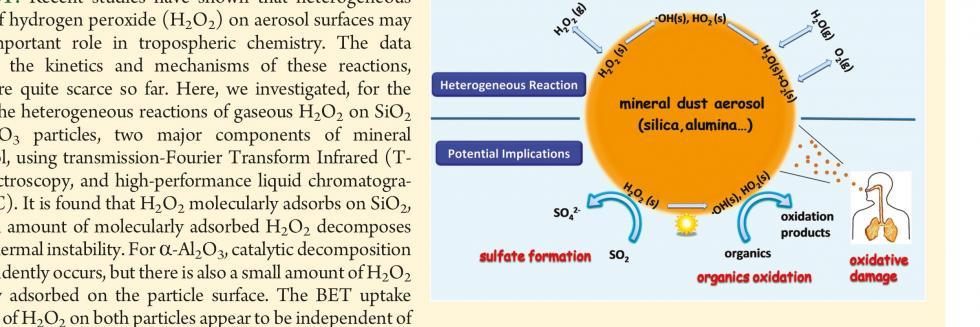

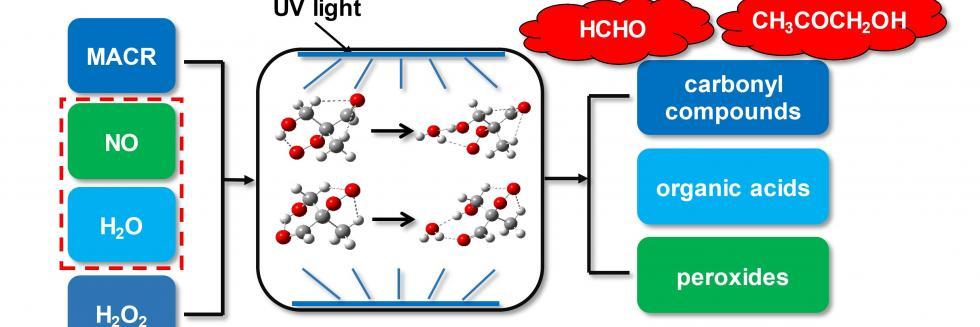
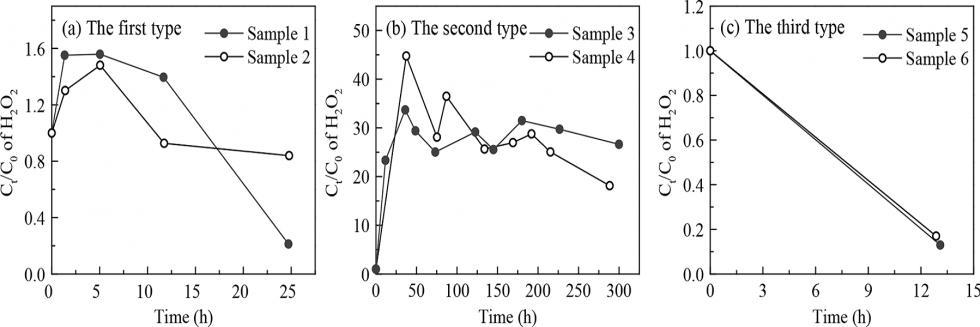
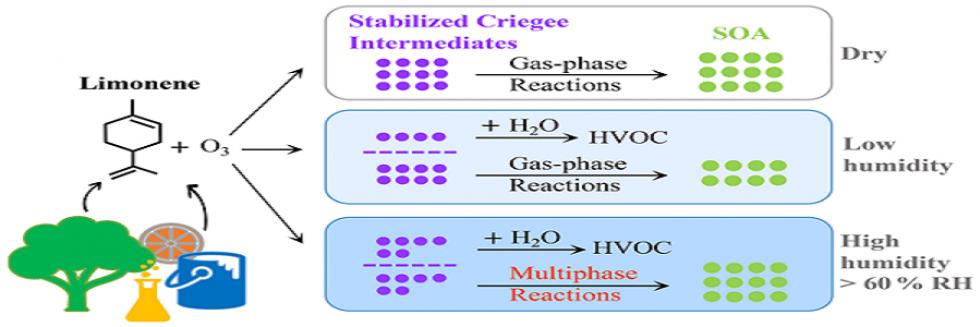
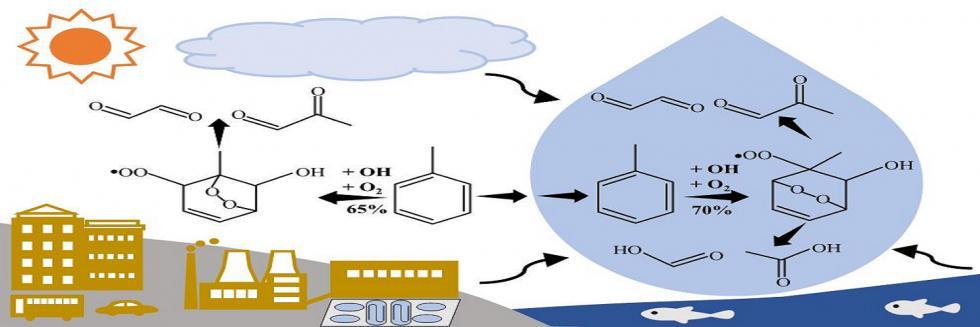
欢迎全国2022级优秀本科生推免本研究组2026级博士生!
欢迎全国2021级优秀本科生推免环境学院2025级研究生!欢迎同学来联系本研究组!
2023年7月5日,北京大学2023年研究生毕业典礼暨学位授予仪式在邱德拔体育馆举行,祝贺本研究组董平同学毕业获得博士学位!