Citation:
Deng N, Li Z, Zuo X, Chen J, Shakiba S, Louie SM, Rixey WG, Hu Y*. Coprecipitation of Fe/Cr Hydroxides with Organics: Roles of Organic Properties in Composition and Stability of the Coprecipitates. Environmental Science &Technology [Internet]. 2021;55:4638-4647.
摘要:
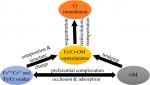
Iron hydroxides are important scavengers for dissolved chromium (Cr) via coprecipitation processes; however, the influences of organic matter (OM) on Cr sequestration in Fe/Cr-OM ternary systems and the stability of the coprecipitates are not well understood. Here, Fe/Cr-OM coprecipitation was conducted at pH 3, and Cr hydroxide was undersaturated. Acetic acid (HAc), poly(acrylic acid) (PAA), and Suwannee River natural organic matter (SRNOM) were selected as model OMs, which showed different complexation capabilities with Fe/Cr ions and Fe/Cr hydroxide particles. HAc had no significant effect on the coprecipitation, as the monodentate carboxyl ligand in HAc did not favor complexation with dissolved Fe/Cr ions or Fe/Cr hydroxide nanoparticles. Contrarily, PAA and SRNOM with polydentate carboxyl ligand had strong complexation with Fe/Cr ions and Fe/Cr hydroxide nanoparticles, leading to significant amounts of PAA/SRNOM sequestered in the coprecipitates, which caused the structural disorder and fast aggregation of the coprecipitates. In comparison with that of PAA, preferential complexation of Cr ions with SRNOM resulted in higher Cr/Fe ratios in the coprecipitates. This study advances the fundamental understanding of Fe/Cr-OM coprecipitation and mechanisms controlling the composition and stability of the coprecipitates, which is essential for successful Cr remediation and removal in both natural and engineered settings.附注:
PMID: 33760589
