摘要:
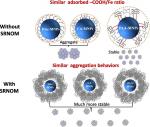
Magnetite nanoparticles (MNPs) with varied organic coatings (OCs) which improved their stability have broad environmental applications. However, the adsorbed amounts and layer thickness of varied OCs onto MNPs during the synthesis were generally not or poorly characterized, and their interactions with natural organic matter (NOM) were still in progress. In this study, acetic (HAc), citric (CA), and polyacrylic acid (PAA) were selected as model OCs, the adsorption behaviors of OCs on MNPs were characterized under varied aqueous C/Fe ratios, and the aggregation behaviors of MNPs with varied OCs (OC-MNPs) at neutral pH (7.0 ± 0.2) with NaCl (5–800 mM) in the presence/absence of NOM were systematically investigated. Under low aqueous C/Fe ratio, the adsorbed amounts of model OCs as –COOH/Fe ratio followed the order: CA ≈ PAA >> HAc. With high aqueous C/Fe ratio, the maximum adsorbed masses of OC-MNPs were similar. The adsorbed layer thicknesses of OC-MNPs were thoroughly characterized using three different methods, all showing that the adsorbed layer of PAA was thicker than that of CA and HAc. Derjaguin–Landau–Verwey–Overbeek (DLVO) and extended DLVO (EDLVO) calculations showed that electrostatic and van der Waals forces were dominant for CA-MNPs and HAc-MNPs stabilization; while steric repulsion played major roles in stabilizing PAA-MNPs, probably due to a thicker PAA layer. In the presence of NOM, stability behaviors of all OC-MNPs were similar, ascribing to the much greater amounts of NOM adsorbed than the OCs, causing greater steric repulsion. This study provides new mechanistic insights which could help better understand the effects of varied OCs on MNPs' colloidal stability.
Link