Citation:
Yang, X. ; Zhang, D. ; Liao, Y. ; Zhao, D. *. Toward an Air-Stable Triradical with Strong Spin Coupling: Synthesis of Substituted Truxene-5,10,15-Triyl. J. Org. Chem. 2020, 85, 5761-5770.
摘要:
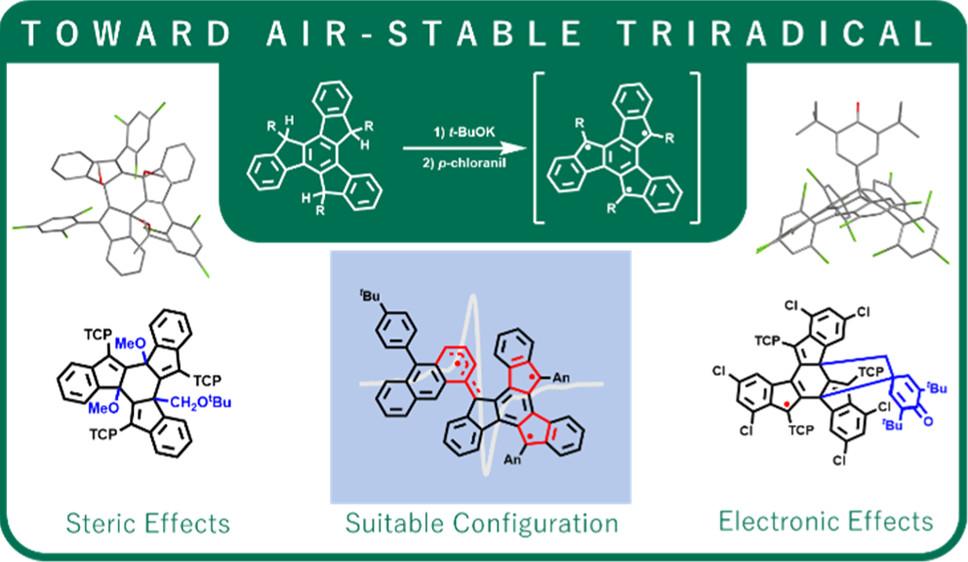
With the aim to achieve air-stable polyradical species manifesting strong spin coupling, synthetic endeavors are made toward triradical molecules featuring a truxene-triyl skeleton. Commonly used steric-hindering side groups such as 2,4,6-trichlorophenyl and 9-anthracenyl are both found to be incompetent at stabilizing the targeted truxene triradical, which appears to be elusive from isolation and characterization. Nonetheless, singlecrystal structures of adducts formed by relevant radicals are obtained, which strongly suggests the transient existence of the designed triradicals. Finally, a truxene triradical comprising 1-anthracenyl along with two 9-anthracenyl substituents is successfully isolated and found to exhibit decent stability in air. We propose that because of the smaller dihedral angle assumed by 1-anthracenyl with respect to the plane of truxene-triyl, more effective pi-conjugation allows the spin density to be more widely delocalized and distributed to the anthracenyl side groups. Thus, higher stability is gained by the triradical molecule.附注:
See also: 2020
